Manufacturing Hope

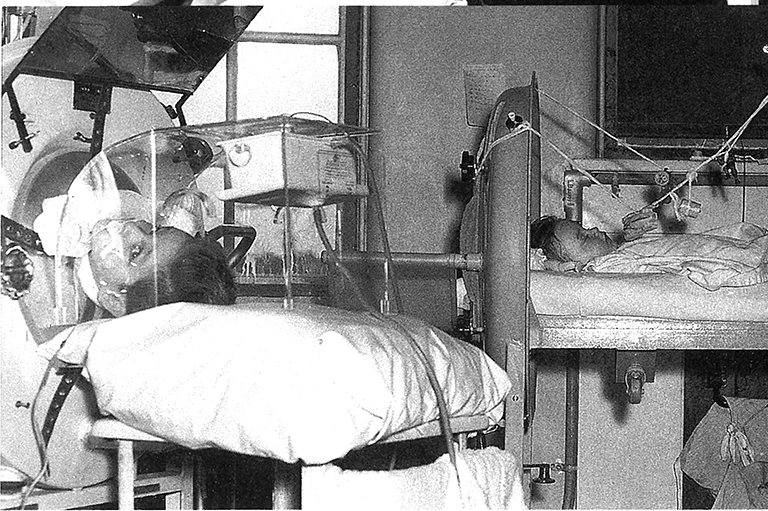
One mild day in the middle of May 1921, two men entered a small, filthy upper-floor room in the University of Toronto’s imposing medical building, on King’s College Circle, and began to scrub every surface. The elder of the pair, Frederick G. Banting, was a twenty-nine-year-old orthopedic surgeon, then based in London, Ontario. The other was Charles H. Best, a twenty-two-year-old physiology and biochemistry student who had just finished his fourth year at the U of T. They had met just days before — introduced by John J.R. Macleod, one of Best’s professors and an eminent U of T scientist.
Months earlier, Banting had cold-called Macleod with an idea for treating diabetes, a debilitating and frequently lethal condition that impairs the body’s ability to metabolize carbohydrates. Scientists had long believed that the pancreas produced a substance that breaks down sugar, allowing it to be converted into energy. Banting, who had no experience in endocrinology, wanted to surgically induce diabetes in laboratory animals by tying off or removing the pancreas and extracting what a later collaborator dubbed the “mysterious” substance. He would then re-administer this secretion to see if the diabetes symptoms — excessive sugar in blood and urine, weight loss, kidney failure, coma — would abate.
With 7 uniquely curated newsletters to choose from, we have something for everyone.
Macleod, a Scottish physiologist with extensive experience in this field, was skeptical but sufficiently interested that he offered Banting a tiny space in which to work, a lab assistant (Best), and access to some of the medical school’s resources.
On May 17, Banting and Best returned to their now-tidied workroom and performed the first operation, on a brown spaniel. For the next three months, the pair carried out numerous such procedures on other lab dogs, testing different ways of reviving the animals with a substance they began to refer to as “isletin.” The summer was sultry; the conditions in their airless space were abysmal. They even had to help to clean the cages of the lab animals in the room next door in order to contain the reek.
Worse, Banting’s scheme did not seem to be working. With a few dogs they observed hints of progress, only to watch as the animals soon died. On August 11, however, they performed the procedure on a yellow collie, identified as “Dog 92” in Banting’s lab journal.
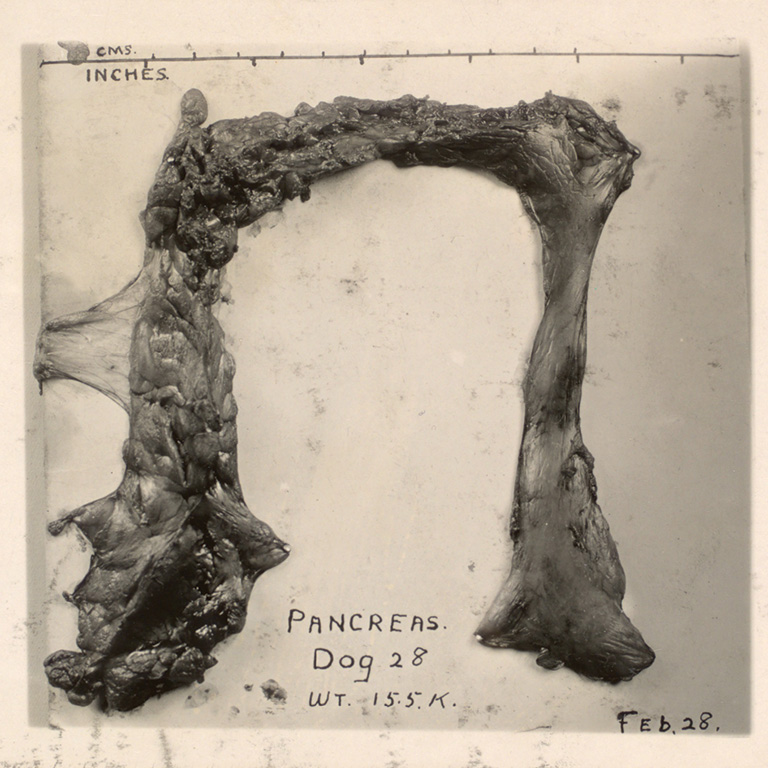
After removing the collie’s pancreas and triggering a diabetic response, they injected it with their isletin serum, and the animal responded immediately. Dog 92 thrived, becoming not only their de facto lab pet but frisky proof that they might be on to something. Yet in late August the collie’s health began to falter as it stopped responding to new forms of the isletin that Banting and Best had produced from cats and rabbits. Finally, on August 31, Dog 92 died.
Weary and frustrated, Banting felt overwhelmed. “I have seen patients die and I have never shed a tear,” he noted in his 1940 memoir, in a passage quoted in Michael Bliss’s fascinating 1982 book, The Discovery of Insulin. “[B]ut when that dog died I wanted to be alone for the tears would fall despite anything I could do.”
Scarcely half a year later, Banting, Best, Macleod, and University of Alberta biochemist James Collip — who had joined the project in the fall of 1921 — were in a position to publish the results of successful early human clinical trials of insulin derived from beef pancreas. Requests for treatment from desperate parents of ailing diabetic children poured in to Toronto as word spread. The drug soon went into mass production, earning, in 1923, a Nobel Prize for Banting and Macleod.
Advertisement
It would be fair to say that insulin is quite possibly Canada’s most consequential scientific achievement, and it’s certainly the discovery that put U of T on the global research map. Now synthesized and produced in various forms, insulin remains the treatment of choice for type 1, or juvenile, diabetes, which is a chronic and possibly genetic condition that begins in childhood. Type 2 diabetes, which typically begins in adulthood and is strongly associated with carbohydrate-rich diets and sedate lifestyles, is likewise treated with insulin but also requires behavioural changes and other forms of glucose monitoring to prevent related illnesses such as stroke, heart disease, blindness, and gangrene in the lower extremities.
An estimated 415 million people worldwide suffer from diabetes (2015 figures), and that number is expected to rise to about 640 million by 2040. The United States alone spends about US$1 billion a year on diabetes research. In Canada, where almost a third of the population either suffers from diabetes or is at risk of developing it, the estimated cost of treatment between 2012 and 2022 will be $15 billion. The direct cost to the health-care system for 2020 alone was $3.8 billion.
Banting and Best’s century-old breakthrough came scarcely two years after the calamitous 1918–20 Spanish flu pandemic. The discovery played a role in the major medical and public-health advances that defined the 1920s, according to the Canadian Public Health Association.

The legacy of their work played out in other ways, too. For example, during a 1923 House of Commons debate on how to recognize the discovery, one Conservative MP, Tommy Church, bruited the idea of federal funding for scientists, something that’s now taken for granted but was unheard of then. Two years later, Banting set up a research foundation that continues to give out grants to scientists. In 1930, the U of T established the Banting and Best Department of Medical Research, on the north edge of the campus, with Best, by then a full professor and medical doctor, as its first director.
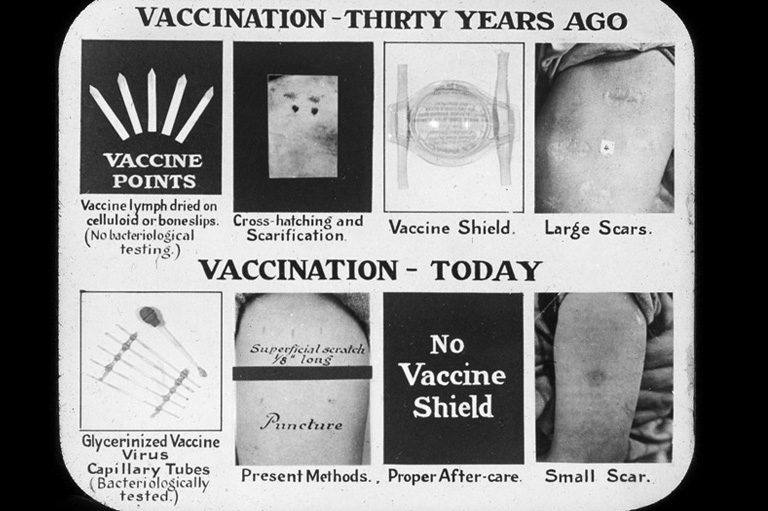
What’s more, the insulin discovery laid the groundwork for two critical developments in the then-nascent pharmaceutical industry. Thanks to an innovative production and intellectual-property partnership between the inventors, the U of T, and the Connaught Medical Research Laboratories — a self-financing arm of the university’s department of hygiene — large quantities of insulin were produced using highly standardized methods to ensure quality and with price controls in place. Both measures meant that diabetics had access to reliable versions of the drug, which they had to take multiple times a day for the rest of their lives. By the 1930s, governments in Canada and the United States began to regulate the testing and production of drugs to ensure safety. The irony is that scientists and pharmaceutical companies today would never be able to develop a drug like insulin in the way that Banting and Best did in 1921.
Yet, for all its many epic elements, the story of insulin, as Bliss showed, involved lots of human drama and a disconcerting amount of chance. At various times, the friction among the four scientists threatened to boil over. Banting almost gave up several times and was angry that the Nobel jury didn’t recognize Best’s work. The project was by no means a predictable march in the direction of a foregone conclusion. Indeed, scientists in Germany and Romania, among other places, were also zeroing in on the same insights that drove Banting and Best.
“What makes this story unique is that there were four people in the right place at the right time, working on the same project,” said Grant Maltman, curator of the Banting House Museum in London, Ontario, which is something of a pilgrimage site for diabetes sufferers and scientists alike. “As Michael Bliss said, Banting and Best in 1921 were leading in a race they didn’t know they were in.”
Save as much as 40% off the cover price! 4 issues per year as low as $29.95. Available in print and digital. Tariff-exempt!

As the urban legend had it, Banting awoke in the middle of the night on October 31, 1920, having literally dreamt up an approach to finding a way of isolating the mysterious pancreatic substance that might cure diabetes. The tale became a fixture in the U of T-fuelled hagiography that grew up around Banting, who died in a 1941 plane crash.
Best went on to become one of the U of T’s stars. “He defined the story for a long time,” said medical historian Christopher Rutty, an adjunct U of T public health professor who has written an account of the discovery for the heritage organization Defining Moments Canada and pharmaceutical company Sanofi Pasteur Canada. “People were afraid to argue with him.”
Shortly before Best’s death in 1978, Michael Bliss, then early in his career as a U of T professor of Canadian history, began to reconstruct the momentous discovery, drawing on lab notes, archival material, and interviews with surviving witnesses and early patients. Bliss, interested from childhood in medicine and eventually the author of three books on medical history, wanted to cut through the mythology in order to tell the insulin story that emerged from primary sources.

Born in the farming community of Alliston, north of Toronto, Banting was a hard-working but not especially brilliant student. He served in the Canadian military during the First World War, gaining extensive experience as a battlefield surgeon.
After the war, he was awarded the Military Cross and worked briefly at Toronto’s Christie Street Veteran’s Hospital but failed to find a permanent position as a surgeon in the city. A mentor suggested that he hang out a shingle in London, Ontario, which worked out because his fiancée lived in nearby Ingersoll.
Banting bought a rambling yellow-brick house near London’s downtown and set up his practice in the summer of 1920. As Maltman noted, his first patient turned up a month later, a man looking for a prescription for alcohol for a friend with a drinking problem. “A great Canadian hero starts off his career as a bootlegger,” he said.
That fall, Bliss wrote, the University of Western Ontario’s medical school offered Banting a gig as a lab demonstrator and occasional lecturer, for two dollars an hour. Towards the end of October, he was asked to deliver a talk on carbohydrate metabolism, a subject about which Banting knew little. The night before the lecture, he found himself reading a medical journal article loosely related to the topic, by Moses Barron, an American pathologist.
The small study described Barron’s observations about what happened when gallstones blocked a pancreatic duct, as well as the relationship between the viability of certain cells, known as the “islets of Langerhans,” in the pancreas and the onset of diabetes. The finding was minor, Bliss noted. “The sole importance of Barron’s article in the history of medicine is that Fred Banting happened to read it in the evening of a day he had been thinking about carbohydrate metabolism.”
Late that night, Banting lay in bed, tossing and turning as he fretted about money and the lecture. As he turned the subject around in his mind, he had a thought, which he jotted in a notebook he kept next to his bed. “Diabetus [sic]. Ligate pancreatic ducts of a dog. Keep dogs alive till acini degenerates leaving Islets. Try to isolate internal secretion of these to relieve glycosurea [excess sugar in the urine].”
“Those fifteen words,” Maltman said, referring to the first sentence, “led to the discovery of insulin.” Banting’s wee-hours inspiration, moreover, reflected his surgical training. After all, the idea turned on a finicky procedure: tying off tiny tubes at one end of a pancake-shaped organ tucked underneath the stomach.
At the time, the state of the art in diabetes treatment involved starvation diets, an approach pioneered by a stern American physician named Frederick Allen. Late in the nineteenth century, quackery prevailed among diabetes “experts,” who claimed they could revive patients from diabetic comas with “cures” ranging from sodium bicarbonate to opium. Some doctors told sufferers to eat as much as possible, but, Bliss wrote, Allen believed in combating the “modern fallacy” that the solution lay in replacing calories lost due to excessive urination, a hallmark of diabetes. His approach: Bring patients, mostly children, to the brink of starvation with strict no-carbohydrate diets that contained well under one thousand calories per day.

Macleod came from a very different camp. A well-regarded scientist who taught at Cleveland’s Western Reserve University before coming to the U of T, he had written a book in 1913 on the physiology of diabetes, concluding that the pancreas produced an “internal secretion” that regulated blood sugar and that was absent in diabetics. Allen, however, dismissed such theories. Macleod himself, Bliss wrote, was skeptical that the secretion could ever be isolated and used as a treatment.
When Banting was casting around for whom to approach about his middle-of-the-night brainstorm, colleagues directed him to Macleod. The young surgeon and the veteran, self-possessed professor — Macleod was forty-six when they first met on November 7, 1920, and “intimidating,” as Rutty observed — had a tumultuous relationship. Macleod agreed to allow Banting to test his hypothesis, partly in order to confirm that the elusive secretion couldn’t be obtained by ligating the pancreas and extracting those cells after it atrophied. Rutty noted that Macleod was impressed by Banting’s surgical skills. “Banting stood a better chance of completing the procedure effectively,” he said. But the young surgeon bristled at the notion that his idea would merely be pressed into service to close off a potential line of scientific inquiry.
At the end of that hot, frustrating summer of 1921, Banting reported the halting progress to date, while Macleod, who had spent those months in Scotland, offered up his own sharp critiques. At one point, Banting brashly threatened to take his research elsewhere if Macleod didn’t provide more resources, including a janitor to clean the animal cages and some kind of stipend for himself. Macleod relented.
Banting and Best also had their moments. Bliss describes one incident when Banting read Best, who was very much the junior partner, the riot act for failing to keep the lab spotless and hygienic. Banting later recounted wondering whether they would come to blows. Best stormed off but ultimately responded by cleaning the equipment and ensuring that their modest facilities were properly maintained from then on. On other occasions, it fell to the younger man to talk Banting down when failures in the lab brought on dark moods and weekends full of hard drinking.

As the fall of 1921 progressed, the two dogged researchers began to see some more encouraging results from their experiments with various permutations and approaches to extracting the mystery secretion. Another U of T professor had secured for Banting a modest teaching gig so he could have a salary. Then, in December, a fourth scientist joined the team — James Collip, an Alberta biochemist who was on sabbatical and happened to be in the city, working at the Toronto General Hospital. It was a crucial turning point.
Collip had developed a strong track record in blood chemistry. He’d heard about Banting and Best’s project, and offered his assistance. Macleod finally agreed to bring him on board so he could apply his skills as a chemist to creating a viable version of the pancreatic extract that was both pure and potent enough to reverse diabetic symptoms.
There had been another highly important development that fall. Banting and Best had spent one afternoon conducting experiments at the Connaught Antitoxin Laboratory, a U of T spinoff located on a sprawling farm north of Toronto.
Founded in 1914 by public health physician John FitzGerald with five hundred dollars in private funding in a basement lab at U of T, Connaught scaled up in 1917, emerging as a non-profit industrial operation geared at producing vaccines for infectious diseases like diphtheria and, later, polio. FitzGerald’s idea was to model the antitoxin lab on France’s Pasteur Institute. By 1921, Banting and FitzGerald were friends. FitzGerald suggested that he and Best come up to Connaught’s farm site to try to produce a version of the extract using calf pancreas — another critical inflection point.
Through late fall 1921 and into January 1922, the results using extracts from beef pancreas (sourced in local slaughterhouses) that had been subjected to increasingly exacting purification techniques yielded ever-improving results on the test animals. Collip applied his chemistry expertise to developing even more refined versions, and Macleod became much more involved in the project. Soon, Banting began to worry that he might be elbowed aside by the more experienced scientists, even as he finalized the first paper to be published about the group’s findings. “Macleod had become the quarterback of the team,” Bliss observed. “Collip seemed to be doing all the running with the ball.” With the prospect of clinical trials on humans now plausible, Banting wanted to make sure he, and not Collip, would be in charge and then badgered Macleod to allow one of them to proceed.
Today’s heavily regulated pharmaceutical industry spends billions of dollars bringing drugs to market — an R&D odyssey that involves in-depth lab and animal testing and then extensive human clinical trials with hundreds and even thousands of carefully screened participants, multiple principal investigators, publication mandates, randomized control tests, and exhaustive regulatory scrutiny.
Most of that infrastructure didn’t exist in 1922 when Banting and Macleod began planning to test their extract on diabetics. The pharmaceutical industry, as such, was in its adolescence, although firms with now-familiar names — Eli Lilly, Wellcome, and Bayer — were operating. Many drugstore medications came from chemists or snake-oil salesmen. There were few controls on production, safety, or marketing claims, and state regulation was a decade off. Remarkably, Banting and Best often tested the toxicity of their own extracts by trying them on one another.
Through another U of T professor, Macleod arranged that the first human subject was an emaciated, nearly comatose fourteen-year-old boy, Leonard Thompson, then a patient on the Toronto General Hospital’s diabetes ward. Thompson’s father, in desperation, gave his permission to allow the team to test the extract on his son on January 11. Though Leonard’s blood sugar dropped somewhat, he showed little signs of improvement — confirmation, as Bliss noted, that this move had been premature, especially in light of the fact that an enterprising Toronto Star reporter heard about the trial and wrote it up. Macleod worried that such coverage would raise expectations among desperate patients and their families before the extract had proven itself.
A few weeks later, they tried again, this time with a purer extract that Collip, the more experienced biochemist, had prepared in his lab. Thompson promptly showed signs of recovery: His blood and urine sugar levels dropped, his energy returned and he began to gain weight. From that moment on, Thompson had to take daily insulin injections in order to survive. (In fact, he lived a relatively normal life for another thirteen years before dying of pneumonia brought on by influenza.)
Away from Thompson’s bedside, however, simmering tensions between Banting, Collip, and Macleod had come to a head. At issue were the twin questions of production and ownership. If they wanted to administer this new elixir to human beings, they’d need to be able to replicate the recipe and then to guarantee a steady supply that was potent, pure, and standardized in its composition. Banting also suspected that Collip might be angling to secure a patent on the extract. (Collip eventually left the project and returned to Alberta.)
Connaught, Banting reckoned, could solve their problem, and he worked his networks to engage FitzGerald’s team. Just days after Leonard Thompson received the second and more successful injections, the four scientists and Connaught signed an agreement of “cooperation” that effectively prevented any of the individuals from claiming intellectual property rights in the extract while also securing a venue where they could produce a consistent insulin supply.
Through the winter and the spring of 1922, a number of other diabetic patients at Toronto General Hospital received injections. Some patients who seemed beyond hope reacted extraordinarily well to the insulin, in several cases emerging from diabetic comas that prompted witnesses to characterize their recoveries as “resurrections.” But the rollout was plagued by what Rutty referred to as periodic insulin “famines” due to inconsistent production at the Connaught lab — a dynamic that inflamed tensions and forced Best to take a more active role in monitoring the lab work.
Meanwhile, word of the discovery was starting to spread in both the popular press, which frequently ran credulous coverage of new miracle cures, and academic circles. Banting and Best published preliminary findings in the Canadian Medical Association Journal, and Macleod lectured at an academic conference in the United States. At one such gathering, an executive of Eli Lilly & Co., the Indianapolis pharmaceutical manufacturer, offered to help to produce the extract at cost.
News of the discovery got a major boost after word got out that Banting had successfully treated Elizabeth Hughes, the teenaged daughter of the U.S. secretary of state. As pleas from other families of desperately ill patients rolled in, the four scientists and U of T officials realized that they needed to pay attention to the intellectual property being created. Banting, Bliss observed, continued to suspect Macleod’s motives but was also wary of putting his name on a potential patent — something doctors did not do at the time in order to avoid conflicts of interest. Universities also looked askance at tying up intellectual property. “The decision to patent insulin was by no means self-evident for the [U of T] discoverers,” wrote Maurice Cassier and Christiane Sinding in a 2008 article in History and Technology, a Routledge journal. “Patenting a medical discovery clashed with the university’s norms, which proscribed the privatization of the results of academic science.”
Advertisement
Macleod, however, began searching for a more public-spirited way of securing the rights to insulin. He knew of an approach devised a few years earlier by the University of Minnesota and the Mayo Clinic, which together had secured patent rights to a thyroid hormone as a means of ensuring that the licensees used consistent production processes and didn’t run up the prices, according to Cassier and Sindling.
On May 30, 1922, the U of T consummated a production agreement with Eli Lilly and also took steps to ensure that the firm would not try to legally and commercially exploit slight refinements of the extract developed in its own labs.
A similar arrangement with the United Kingdom-based Medical Research Council soon followed. Indeed, as demand for the extract widened, the U of T’s newly established “insulin committee” set to work finding other international academic partners willing to establish local production deals in order to increase access to the extract. By the end of 1922, Best, Banting, and Collip assigned their United States and Canadian patent rights to the U of T for the nominal fee of one dollar — a remarkably altruistic gesture that would be almost unimaginable in today’s profit-driven drug-discovery world. Royalties, in turn, flowed to Connaught and paved the way for its evolution into a major player in Canada’s emerging pharmaceutical sector. Another landmark drug, the anti-coagulant heparin, also came out of Connaught’s labs. Connaught was eventually sold to a federal agency in 1972 and changed hands several times before becoming part of Sanofi Pasteur. Proceeds from the original sale, twenty-nine million dollars, seeded a U of T research fund that continues to dispense grants to this day. “[Macleod] flipped that patent model on its head,” Rutty said. “They were very selfless about it all.” Maltman added: “A hundred years later we have better insulin, but nothing better than insulin.”
The fact that the centennial of the 1918–20 Spanish flu pandemic, today’s COVID-19 pandemic, and the centennial of the discovery of insulin roughly coincide isn’t merely a chronological nicety. The COVID-19 pandemic, according to a literature review compiled by Diabetes Canada, has revealed how type 2 diabetes is a “major co-morbidity” that exacerbates the symptoms of the coronavirus, which has had a disproportionate impact on low-income, racialized, and Indigenous communities.
Epidemiologist Seema Nagpal, Diabetes Canada’s vice-president for science and policy, says the pandemic serves as a reminder that Canada needs to place a greater focus on chronic diseases as part of any future pandemic-preparedness strategy. She and others point out that one of the long-term legacies of the discovery of insulin is that Canada “can punch higher than its weight class” when it comes to diabetes research, especially research into the causes and treatment for type 2 diabetes, itself an epidemic. “The challenge,” Nagpal said, “is that there’s no one solution that will help attenuate type 2 the way insulin helped with type 1.” Nagpal cites two notable Canadian examples: a game-changing discovery by U of T professor Daniel Drucker, who invented a way of boosting a gut hormone linked to insulin production, and which is now used worldwide, and the development at the University of Alberta of the so-called Edmonton Protocol, which sets out methods for transplanting pancreatic islets that govern insulin production in type 1 diabetes patients, allowing them to become insulin-independent.
Technologies developed elsewhere — for example, continuous glucose monitoring — have also played a huge role in improving the quality of life for people with diabetes. And there’s extensive research into not only understanding the development of type 2 diabetes but also early warnings that can be detected through various forms of high-sensitivity blood tests, said Paul Yip, an associate professor of biochemistry at the U of T and a researcher at Toronto’s Sunnybrook Health Sciences Centre. The most recent development, he adds, is the pairing of portable glucose monitors linked electronically to wearable insulin pumps. “What you have is an artificial pancreas.” Bioengineering holds out an even more dramatic outcome. If stem cell research aimed at transplanting islets of Langerhans into a diabetic patient to re-generate the pancreas works, Yip added, “that will essentially be a cure.” Indeed, in November 2020, a U of A research team led by Dr. James Shapiro, who invented the Edmonton Protocol, announced that it had reversed diabetes in mice using stem cells, a development that points to a possible cure.
Back at the Banting House in London, Ontario, Grant Maltman has watched as visitors from all over the world enter the bedroom where the famous Canadian inventor jotted down his middle-of-the-night idea a hundred years ago. Many are diabetics who have come to pay homage. Others are scientists and medical researchers, there to touch the bed frame in reverence — and, possibly, to try to tap in to whatever inspired Banting so many years ago. “Almost to a person,” he said, “they will lean against the bed. You can see the finish is gone on the footboard” where people have touched it. And, when one of them does find the cure, the “Flame of Hope” that burns on a ceremonial pedestal just outside the house will officially be extinguished.
In today’s environment of misinformation and disinformation, it can be hard to know who to believe. At Canada’s History, we tell the true stories of Canada’s diverse past, sharing voices that may have been excluded previously. We hope you will help us continue to share fascinating stories about Canada’s past, highlighting our nation’s diverse past by telling stories that illuminate the people, places, and events that unite us as Canadians, and by making those stories accessible to everyone through our free online content.
Canada’s History is a registered charity that depends on contributions from readers like you to share inspiring and informative stories with students and citizens of all ages – award-winning stories written by Canada’s top historians, authors, journalists, and history enthusiasts. Any amount helps, or better yet, start a monthly donation today. Your support makes all the difference. Thank you!
Themes associated with this article
Advertisement